
Marksans Pharma Ltd.'s wholly owned subsidiary Relonchem Ltd. has received marketing authorisation for two products from the UK regulator, the Medicines and Healthcare Products Regulatory Agency.
On Tuesday, the company declared to exchanges that it had been granted approval for marketing its Rasagiline Relonchem and Olmesartan film-coated tablets.
These tablets will be sold as Rasagiline Relonchem 1 mg, Olmesartan 10 mg, Olmesartan 20 mg and Olmesartan 40 mg.
Rasagiline and olmesartan are drugs that are used to treat Parkinson's disease and hypertension, respectively.
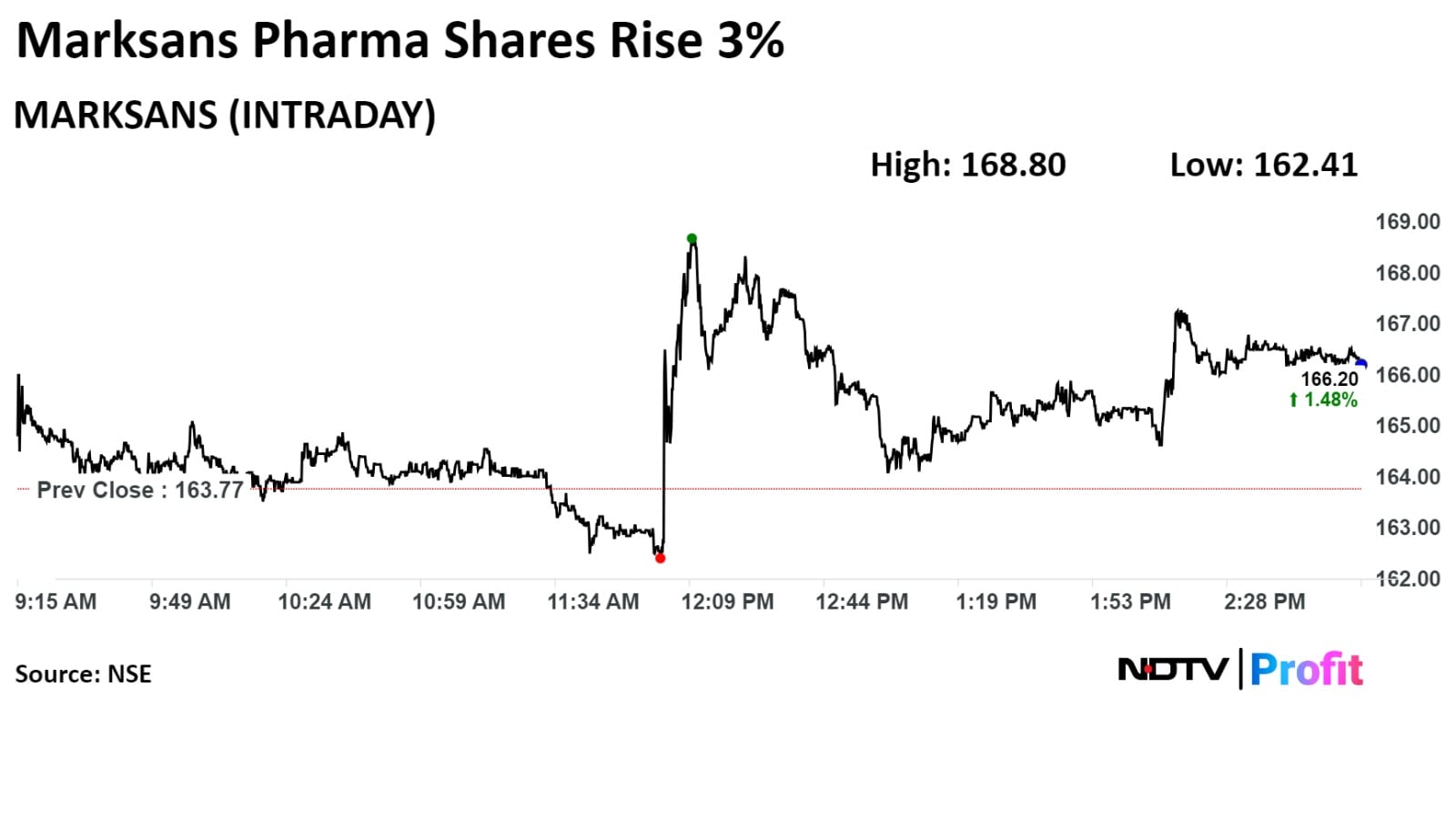
Marksans Pharma shares were trading higher after the UK MHRA granted marketing authorisation for four products to its subsidiary.
The company's stock rose as much as 3.07% during the day to Rs 168.80 apiece on the NSE. It was trading 1.66% higher at Rs 166.34 apiece, as compared to a 0.01% decline in the benchmark NSE Nifty 50 as of 2:50 p.m.
It has risen 79.27% in the last 12 months and 3.35% on a year-to-date basis. The total traded volume so far in the day stood at 1.7 times its 30-day average. The relative strength index was at 58.42.
Four analysts tracking the company have a 'buy' rating on the stock, according to Bloomberg data. The average of 12-month analyst price targets implies a potential upside of 19.4%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























