
Shares of Cipla Ltd. fell 3% on Monday to the lowest level in over two weeks after it received six observations for its manufacturing facility in Goa from the US Food and Drug Administration.
The FDA conducted an inspection at the facility in Goa from June 10–21. The pharma company received the inspectional observations in Form 483, according to an exchange filing.
The form notifies the management about objectionable conditions that prevail in the production process. At the end of the inspection, form 483 is presented and discussed with the senior management to ask them to fill up with corrective measures and implement it, according to the FDA website.
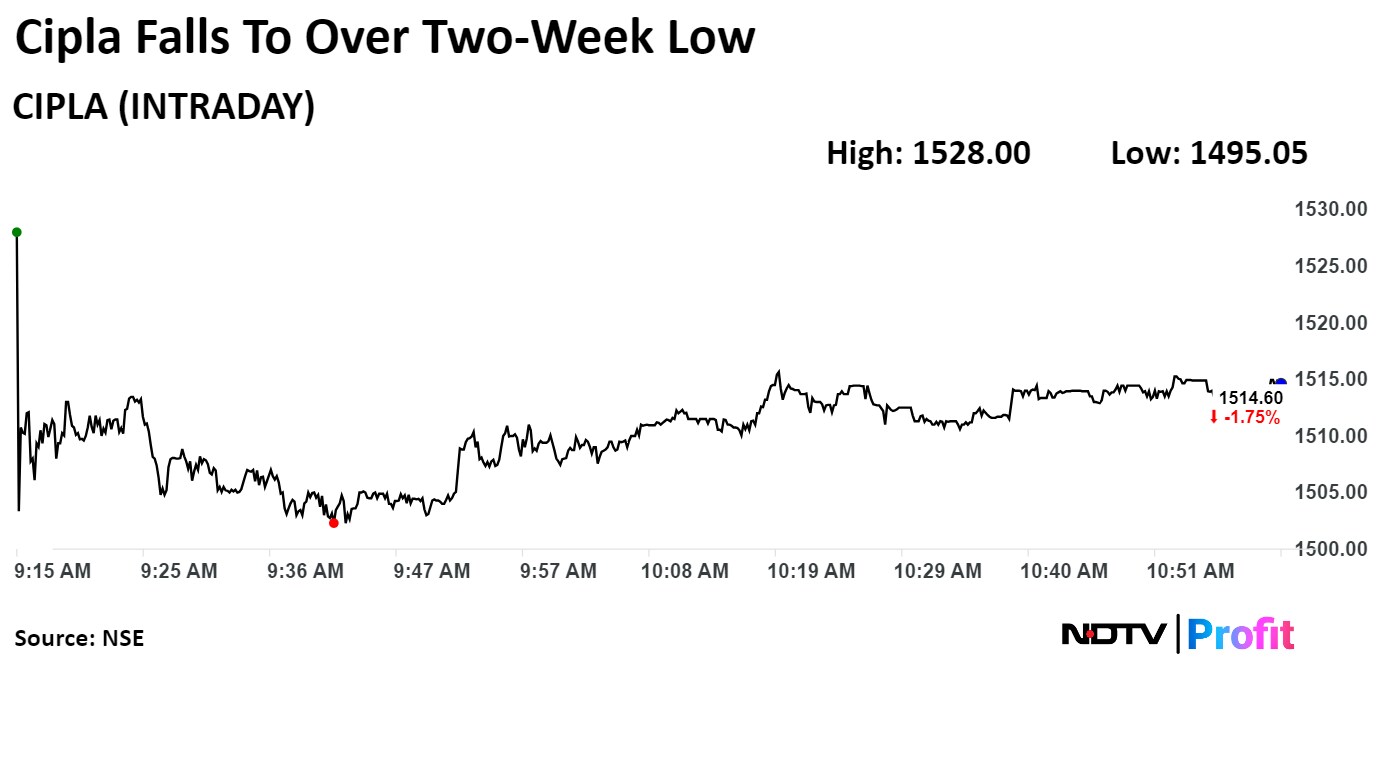
On the NSE, Cipla's stock declined as much as 3.02% during the day to Rs 1,495.05 apiece, the lowest since June 7. It was trading 1.79% lower at Rs 1,513.90 per share, compared to a 0.05% advance in the benchmark Nifty at 11:09 a.m.
The share price has gained 21.5% on a year-to-date basis and 49.19% in the 12 months. The total traded volume so far in the day on NSE stood at 0.26 times its 30-day average. The relative strength index was at 53.33.
Twenty-five out of the 38 analysts tracking the company have a 'buy' rating on the stock, seven recommend 'hold' and six suggest 'sell', according to Bloomberg data. The average of 12-month analyst price targets implies a potential downside of 1.4%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.





















:max_bytes(150000):strip_icc():focal(722x523:724x525):format(webp)/Savannah-Guthrie-mother-Nancy-Guthrie-020226-02-0368cb6ddb864bf09919fd100fe546b0.jpg?im=FeatureCrop,algorithm)

