
Shares of Alembic Pharmaceuticals Ltd. jumped over 7% on Tuesday after it said it got a total of 196 product approvals from the US drug regulator in the third quarter.
The company said its cumulative total of 196 ANDA approvals includes 170 final approvals and 26 tentative approvals from the US Food and Drug Administration during the quarter ended Dec. 31, according to an exchange filing.
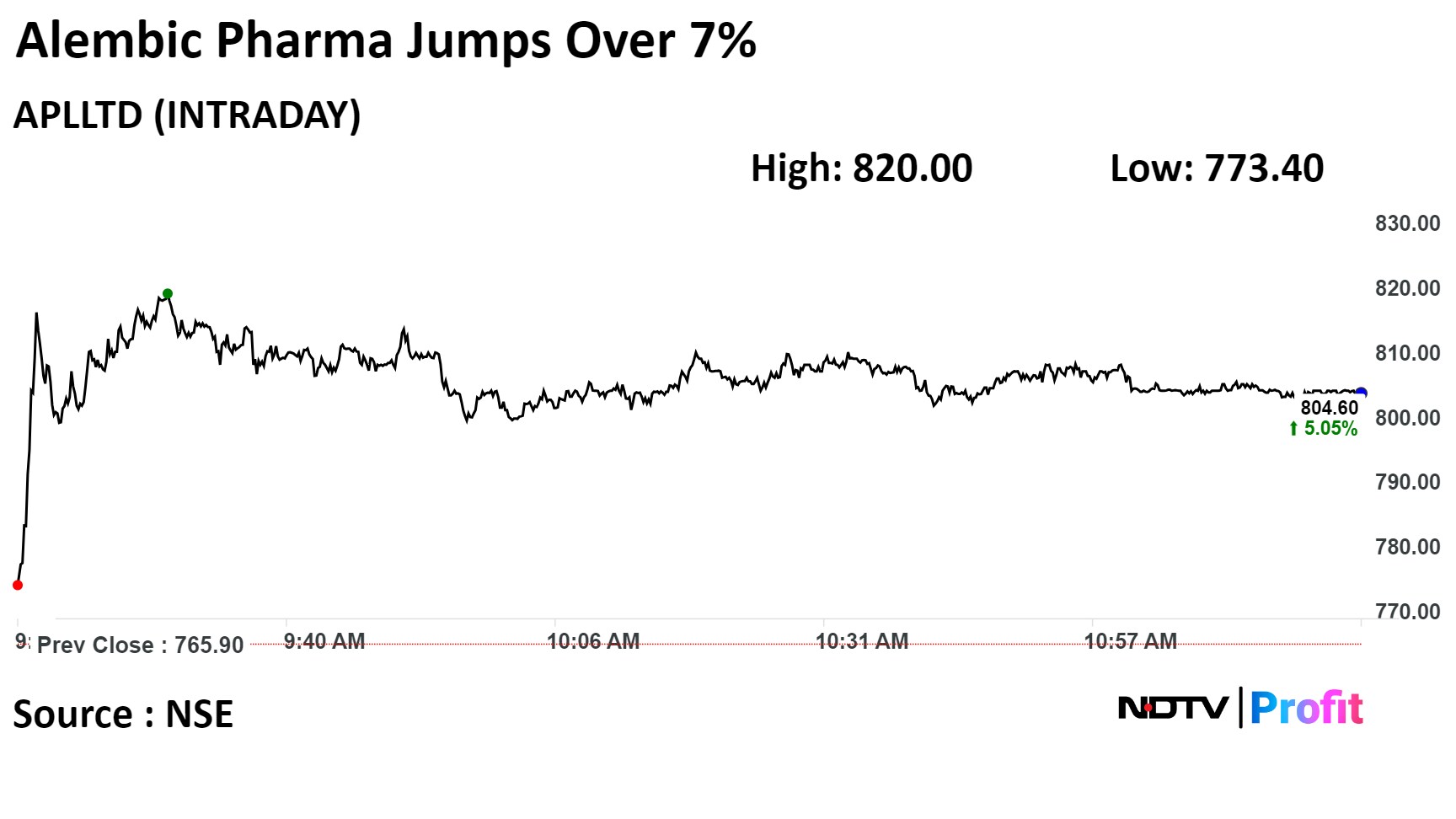
Alembic Pharmaceuticals' stock rose as much as 7.17%, the highest level since Nov. 30, before paring gains to trade 5.16% higher as of 12:02 p.m. This compares to a 0.6% decline in the NSE Nifty 50.
It has risen 41.57% year-to-date. Total traded volume so far in the day stood at 9.9 times its 30-day average. The relative strength index was at 65.72.
Of the 19 analysts tracking the company, eight maintain a 'buy' rating, six recommend a 'hold', and five suggest a 'sell', according to Bloomberg data. The average 12-month analysts' consensus price target implies a downside of 5.9%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























