
Shares of Laurus Labs Ltd. declined nearly 4% on Wednesday as the company received five observation from the U.S. Food and Drug Administration after inspection at its Andhra Pradesh unit.
The regulator inspected Laurus Synthesis Pvt. manufacturing facility in Parawada, Anakapalli, near Visakhapatnam from Dec. 4 to Dec. 12. After inspection, the pharmaceutical company received form 483 with five observations.
Laurus Labs has said it will address the observations within stipulated time.
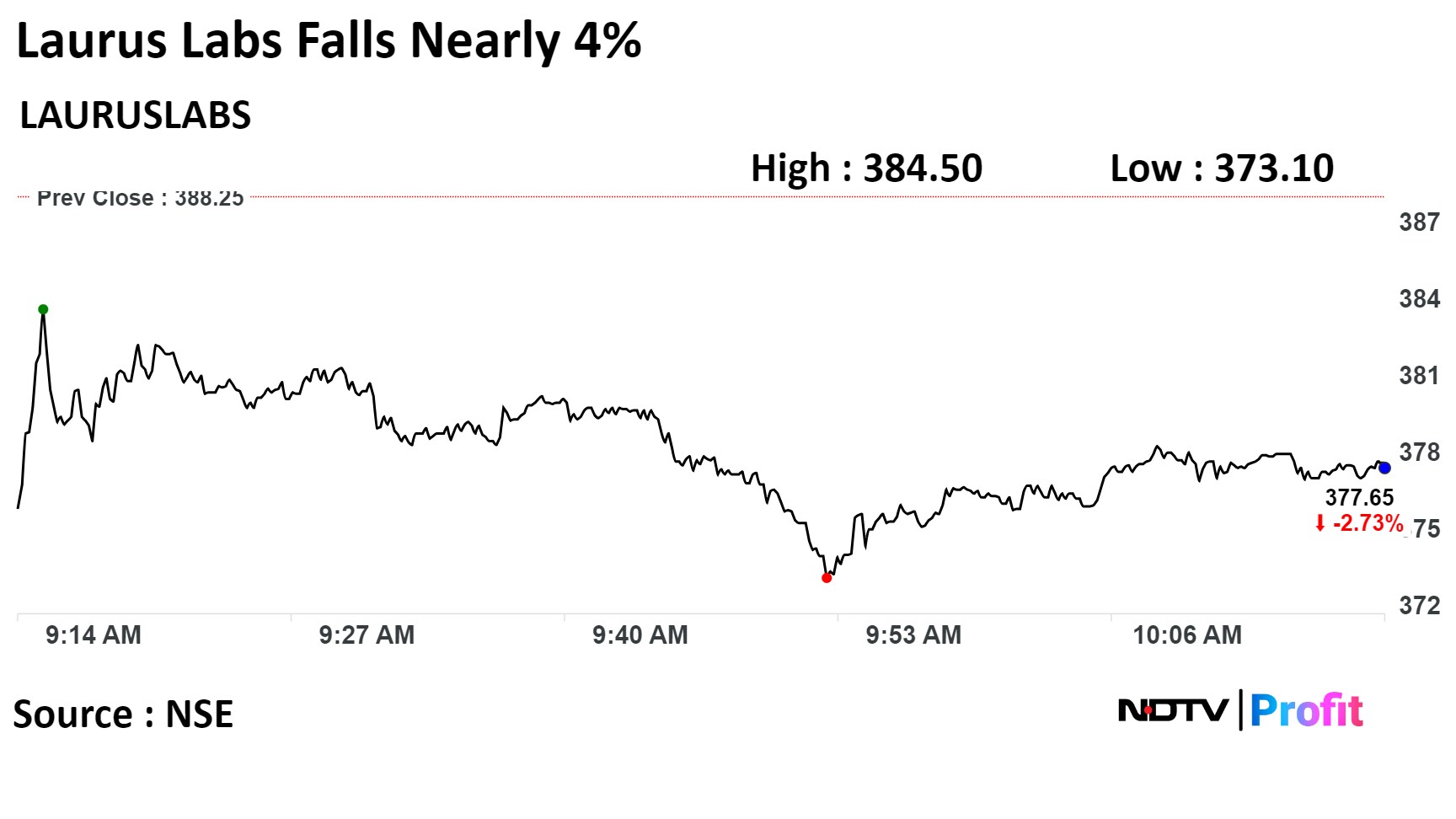
Shares of the company slumped 3.90%, the lowest since Dec. 7, before paring gains to trade 2.95% lower at 10:01 a.m. This compares to a 0.33% decline in the NSE Nifty 50.
The stock has risen 0.44% year-to-date. Total traded volume so far in the day stood at 4.4 times its 30-day average. The relative strength index was at 48.49.
Of the 15 analysts tracking the company, nine maintain a 'buy' rating, two recommend a 'hold,' and four suggest a 'sell', according to Bloomberg data. The average 12-month analysts' consensus price target implies an upside of 2.9%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.




















