
Shares of Glenmark Pharmaceuticals Ltd. rose to a record high on Monday after its unit launched a new eye allergy drug in the US.
Glenmark Therapeutics Inc., USA, has introduced Olopatadine Hydrochloride Ophthalmic Solution USP, 0.1% (OTC) in the American market. This eye allergy drug aims to compete with Pataday Twice Daily Relief, which has seen annual sales of approximately $26.4 million, according to Nielsen data, the company informed in an exchange filing.
Fabio Moreno, head of OTC Sales & Marketing at Glenmark Pharmaceuticals Inc., highlighted the launch as a significant step in meeting the increasing demand for new options in this category.
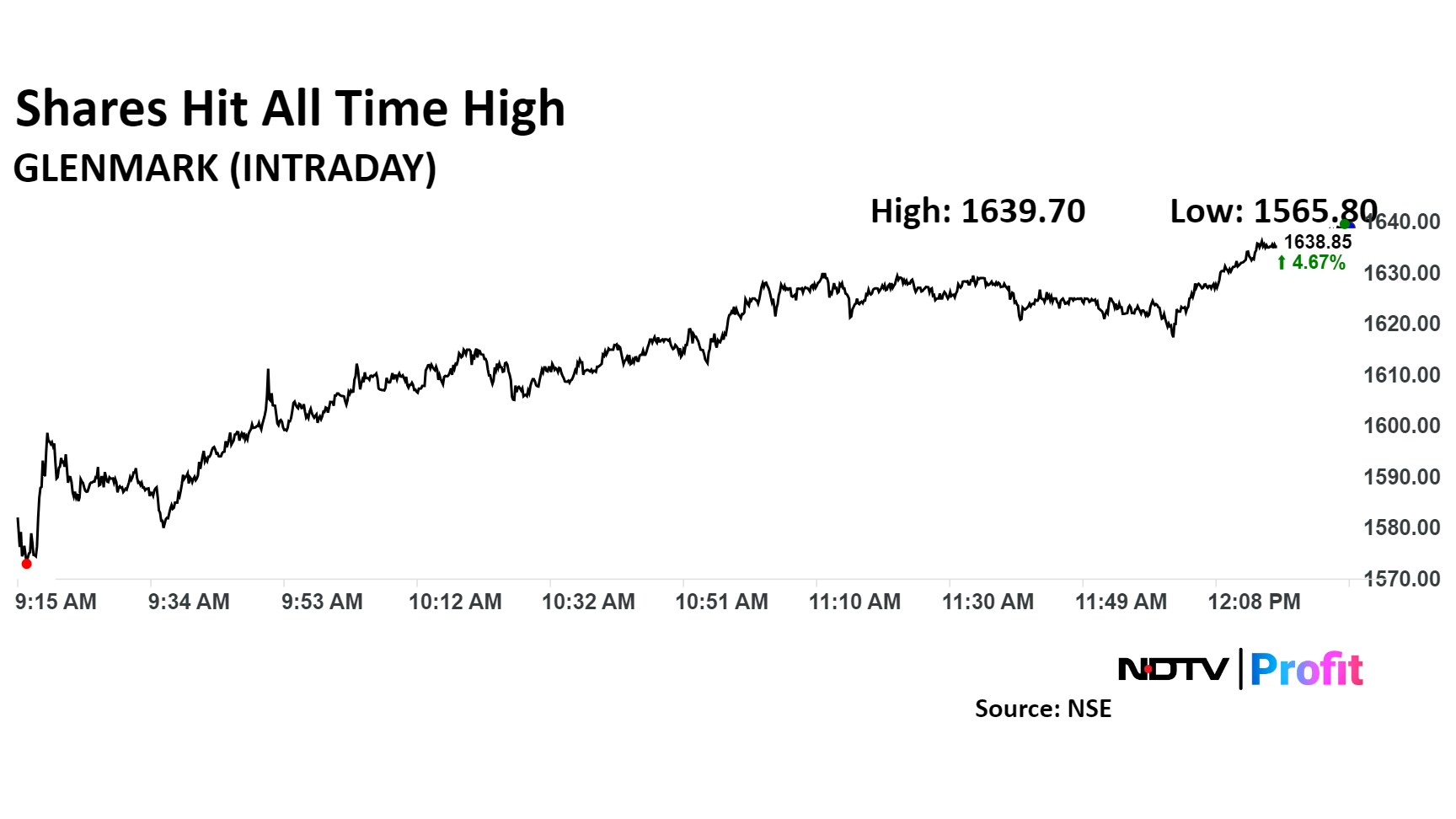
Shares of the company rose as much as 4.51% to 1,636.40 apiece reaching all time high. It pared gains to trade 4.37% higher at Rs 1,634.25 apiece, as of 12:20 p.m. This compares to a 0.40% advance in the NSE Nifty 50 Index.
The stock has risen 113.72% in the last 12 months and 91.74% year-to-date. Total traded volume so far in the day stood at 2.6 times its 30-day average. The relative strength index was at 81, signalling the stock is overbought.
Out of 12 analysts tracking the company, seven maintain a 'buy' rating, three recommend a 'hold,' and two suggest a 'sell,' according to Bloomberg data. The average 12-month consensus price target implies an downside of 4.8%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























